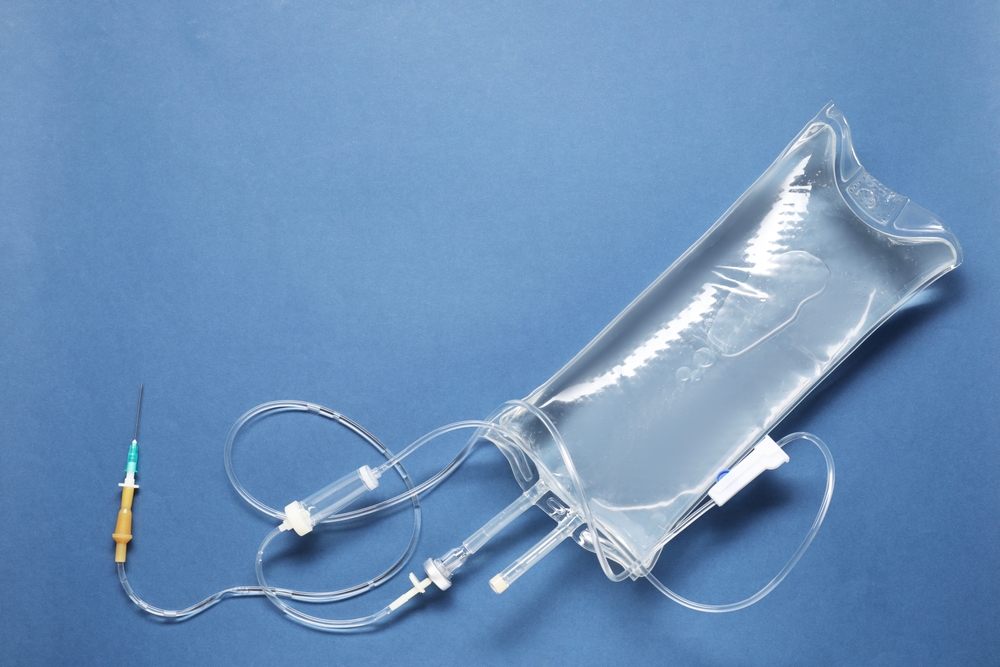
At Chemique Pharmaceuticals Inc., we are committed to redefining patient safety and operational efficiency in hospital and clinical settings. One of the most critical advancements we’ve made toward this goal is the development of our pre-spiked IV bags. These pharmacy-grade infusion bags, precisely prepared in ISO-5 cleanroom environments, are engineered to ensure a pyrogen-free infusion process, dramatically reducing the risk of contamination, enhancing sterility assurance, and simplifying administration workflows across healthcare facilities.
Elevating Sterile IV Compounding Standards
Sterile IV compounding is the cornerstone of safe intravenous therapy, particularly when dealing with high-risk medications or total parenteral nutrition (TPN). Chemique Pharmaceuticals Inc. understands that sterility is non-negotiable. That’s why our pre-spiked IV bags are produced under rigorous aseptic conditions that fully comply with USP 797 guidelines. These industry standards define stringent parameters for environmental monitoring, personnel hygiene, and equipment maintenance within sterile compounding areas.
Our pre-spiked bags are compounded inside ISO-5 cleanrooms—controlled environments where the air quality meets the highest standard for particulate count and microbial contamination. These rooms maintain over 99.99% air purity through the use of HEPA filtration systems, minimizing airborne contaminants and supporting the manufacture of sterile products with unmatched precision.
By producing in ISO-5 conditions, we ensure that our pre-spiked bags are pyrogen-free, eliminating one of the leading causes of infusion-related complications: endotoxin contamination. Endotoxins, often introduced through microbial contaminants, can provoke severe immunological reactions, making pyrogen control essential for patient safety.
Infection Risk Reduction with Pyrogen-Free Infusion
The safety profile of IV therapy hinges heavily on controlling infection vectors. Traditional IV bag preparation typically involves manual spiking at the bedside or in the pharmacy. This process, even with trained personnel, introduces multiple opportunities for contamination—from compromised technique to breaches in sterility due to rushed environments or inadequate equipment sterilization.
Our pre-spiked IV bags are engineered to address these vulnerabilities. Each bag is aseptically spiked and sealed during production, thereby eliminating the need for end-user manipulation. This sealed system not only prevents the ingress of pathogens but also upholds the sterility of the fluid pathway from manufacturing to patient administration. The result is a pyrogen-free infusion process that is reproducible, reliable, and free from the inconsistencies of manual preparation.
Moreover, our sterility assurance is further validated by regular microbial testing and adherence to GMP (Good Manufacturing Practice) protocols. Chemique Pharmaceuticals Inc. maintains strict batch-release criteria to ensure that every pre-spiked IV bag leaving our facility is safe, effective, and fully compliant with regulatory standards.
Streamlining Clinical Workflows and Saving Valuable Time
In healthcare settings where every second counts, operational efficiency is just as crucial as clinical efficacy. Nurses and pharmacists are often burdened with the time-consuming task of manually preparing and spiking IV bags—a process that demands meticulous attention to detail and diverts focus from direct patient care.
Our pre-spiked IV bags are designed to remove this bottleneck. By delivering ready-to-use, sterile IV compounding solutions, we empower clinical teams to redirect their efforts toward what matters most: patient outcomes. With our pharmacy-grade infusion bags, nursing staff no longer need to spend valuable minutes preparing infusions, checking for contamination, or ensuring the sterility of makeshift setups. Instead, they can connect the IV bag and proceed with confidence, knowing that the product meets the highest standards for quality and safety.
This time-saving innovation is especially impactful in high-volume settings such as emergency departments, intensive care units, and oncology centers, where the speed of treatment initiation can directly influence patient recovery and prognosis. Additionally, our streamlined delivery model supports just-in-time inventory management, further enhancing efficiency and reducing waste associated with unused or improperly stored components.
Ensuring TPN Safety and Specialized Compounding
Total parenteral nutrition (TPN) presents unique challenges that go beyond routine IV therapy. The complexity of TPN formulations, combined with the heightened vulnerability of the patients who receive them, demands an uncompromising approach to sterility and accuracy. At Chemique Pharmaceuticals Inc., our pre-spiked IV bags offer a safe and reliable solution for TPN delivery.
Each bag is compounded under USP 797 conditions using automated systems that minimize human error and ensure consistent formulation accuracy. Whether delivering amino acids, lipids, electrolytes, or micronutrients, our sterile IV compounding process preserves the stability and integrity of these critical nutrients.
TPN patients often suffer from weakened immune systems, making them especially susceptible to infections caused by endotoxins and particulates. Our commitment to producing pyrogen-free infusion solutions within ISO-5 cleanrooms ensures that TPN safety is never compromised. Through rigorous in-process controls and endotoxin testing, we uphold the trust that healthcare providers place in our products for their most vulnerable patients.
Furthermore, our customizable pre-spiked bag options allow facilities to meet individual patient requirements while maintaining consistent product quality. This adaptability ensures that even the most specialized nutritional needs can be met without sacrificing safety, sterility, or efficiency.
A Future-Focused Commitment to Pharmaceutical Excellence
As healthcare continues to evolve, the demand for high-quality, ready-to-use sterile infusion products is only increasing. At Chemique Pharmaceuticals Inc., we are not just meeting this demand—we are setting the standard. Our pre-spiked IV bags exemplify our commitment to pharmaceutical excellence by integrating advanced cleanroom technologies, robust quality control systems, and a deep understanding of clinical workflow needs.
Each component of our process—from raw material sourcing to final packaging—is scrutinized to ensure alignment with USP 797 requirements and ISO-5 cleanroom best practices. This holistic approach guarantees a level of sterility and reliability that few other manufacturers can offer.
Moreover, our ongoing investment in research and development ensures that we remain at the forefront of sterile IV compounding innovation. We continuously refine our manufacturing techniques and explore novel materials and designs that further enhance the safety and usability of our infusion products.
By choosing Chemique Pharmaceuticals Inc., healthcare providers gain a trusted partner in delivering pyrogen-free infusions that not only protect patients but also elevate the standards of care. Our pre-spiked IV bags are more than a convenience—they are a strategic asset for any facility striving for excellence in infection control, operational efficiency, and therapeutic precision.
Conclusion
Our pre-spiked IV bags reflect the synthesis of safety, efficiency, and innovation. Prepared in ISO-5 cleanroom conditions and compliant with USP 797, they offer a pyrogen-free, pharmacy-grade solution to the modern challenges of IV therapy and TPN safety. At Chemique Pharmaceuticals Inc., we are proud to lead the industry with products that not only meet today’s healthcare demands but also pave the way for a safer, more efficient future.

